You can drag ‘start’ and ‘endpoint.’
Thermodynamics of Gas
The gas changes its temperature while exchanging thermal energy with the outside and sometimes does work outside as it expands or contracts.
The process of changing the temperature, pressure, and volume of the gas is the gas’s thermodynamics. These variables will affect other variables.
We can find many examples of gas thermodynamics around us.
- Sudden explosion occurs inside the engine of the car. At this time, the suddenly inflated gas drives the engine of the car.
- The refrigerator suddenly expands the compressed gas and lowers the internal temperature.
- Rising atmospheres such as cyclones produce clouds as the temperature falls below the dew point.
- The gas tank gets colder while using the portable gas burner.
- The diesel engine compresses the air rapidly to obtain a temperature above the ignition point.
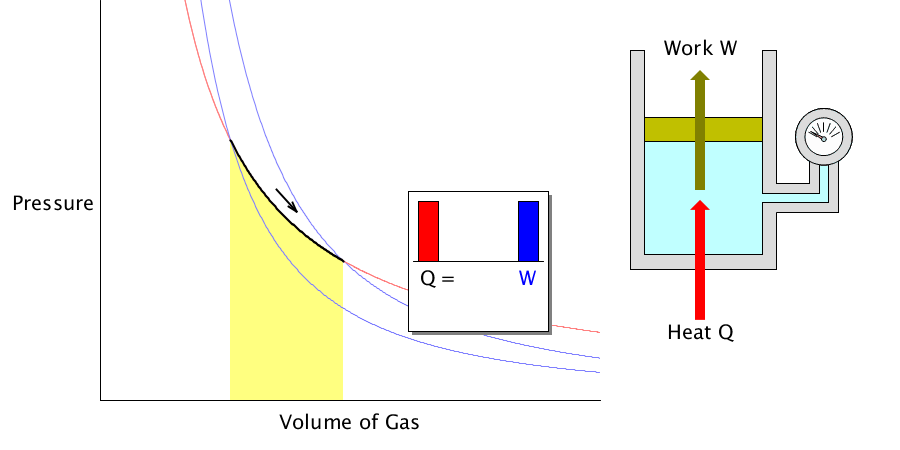
Isothermal process
Because the gas’s temperature is constant, all the heat applied to the gas or absorbed is used to change the volume.
During the isothermal process, the applied heat is converted to 100% work.
In this case, it is assumed that the gas can expand or contract freely.
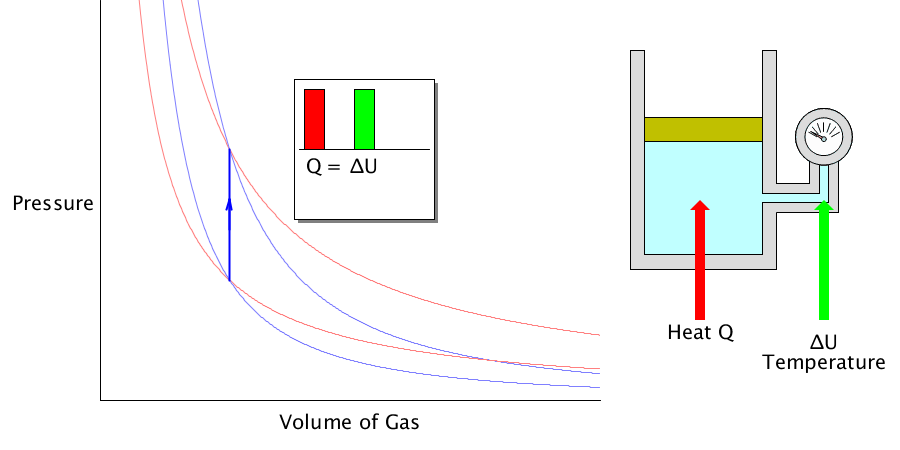
Isochoric process
Since the volume does not change, the amount of work done to the outside becomes ‘0’. The heat energy absorbed or released is used to change the internal energy of the gas.
It does not stretch or shrink. It only changes the temperature of the gas.
In this case, it is assumed that the gas is contained in a solid container, the volume does not change, and the container does not exchange heat with the gas.
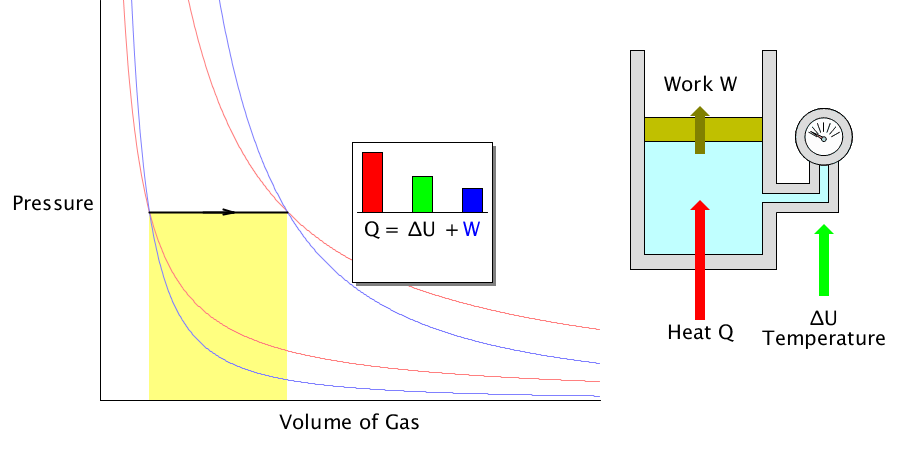
Isobaric process
It is a process in which the volume changes as the ideal gas pressure is maintained.
In the isobaric process, the heat transferred to the gas works outside and changes the system’s internal energy (temperature).
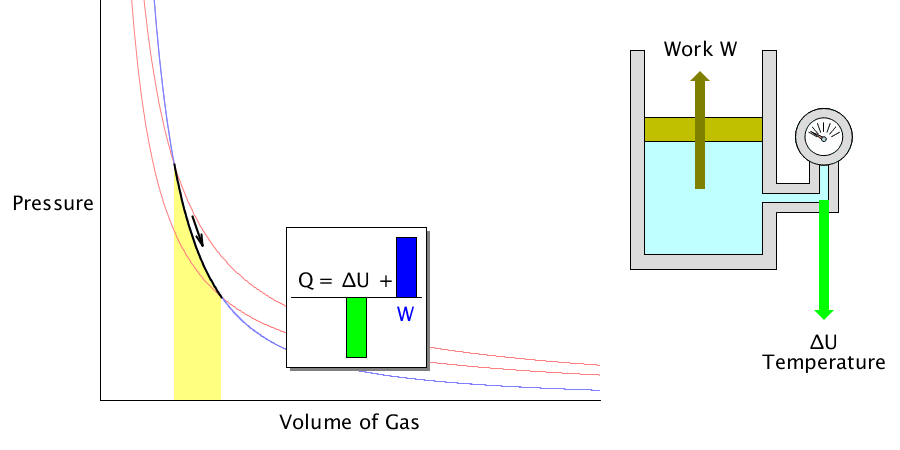
Adiabatic process
It is the process of changing the temperature of the gas when the volume of an ideal gas is expanded or compressed when there is no heat energy exchange from the outside.
For example, suddenly expanding the compressed coolant inside the refrigerator will reduce the temperature.