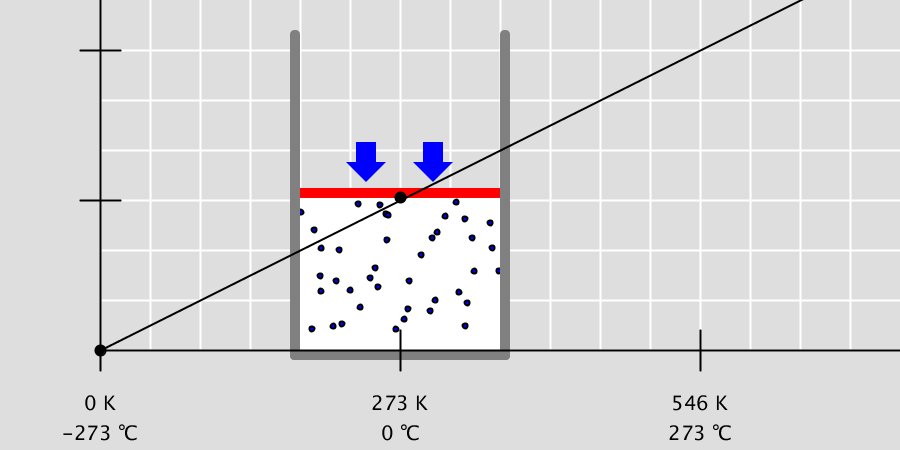
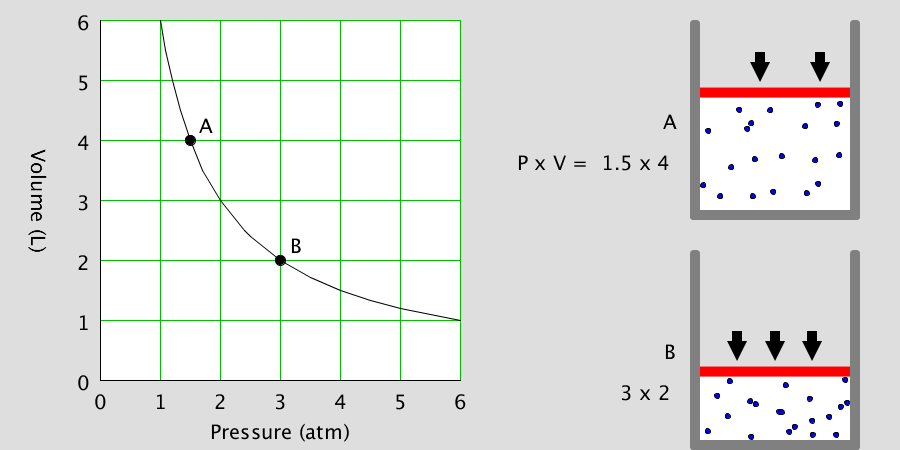
Ionic Bond
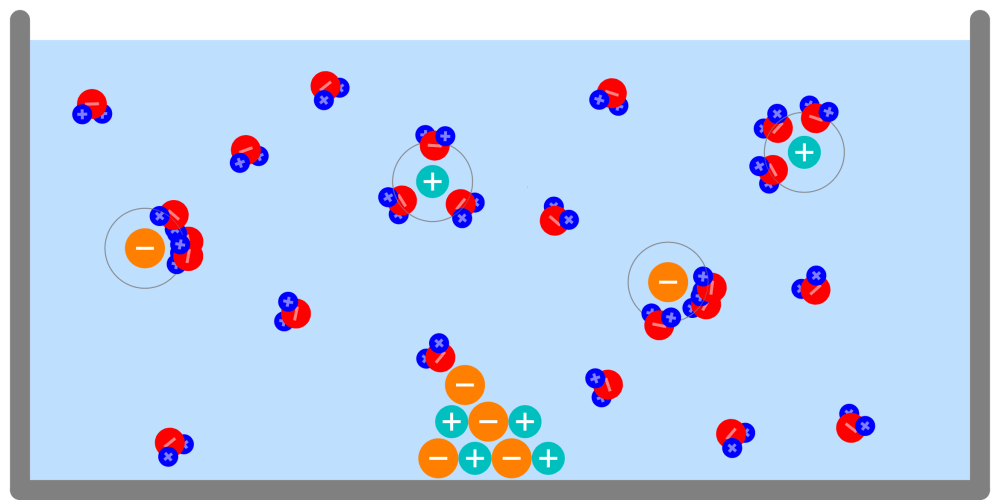
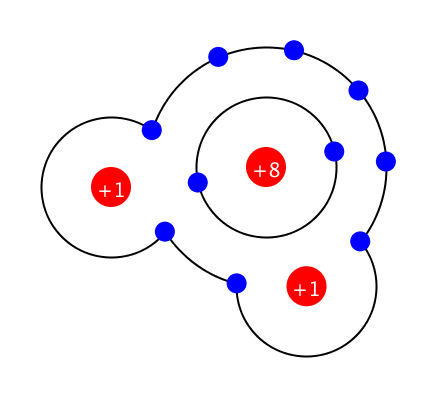
Formation and energy of the ionic bonds Metal atoms have strong properties to lose electrons and become cations. On the contrary, non-metallic atoms have strong properties to obtain electrons and become anions. As these two particles … more