Heat Simulation
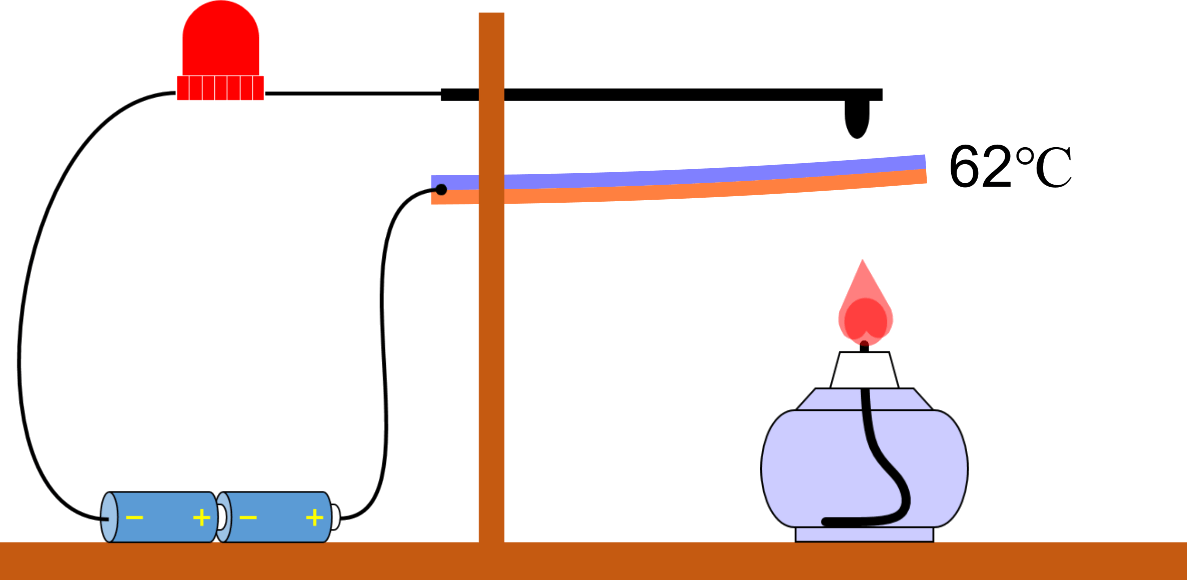
Heat conduction (a good thing about wooden pot handles)
Conduction The ‘conduction’ of heat is how heat is transferred by moving particles that make up an object to neighboring particles. Heat can be transferred even if the particles do not move, mainly by vibration or … more
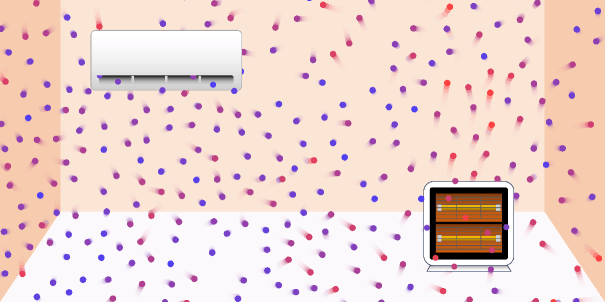
Convection in the room: air conditioners and stoves
Convection in the room There are three ways to transfer the heat: conduction, convection, and radiation. Convection is the method by which heat is transferred by the direct movement of particles that make up a liquid … more
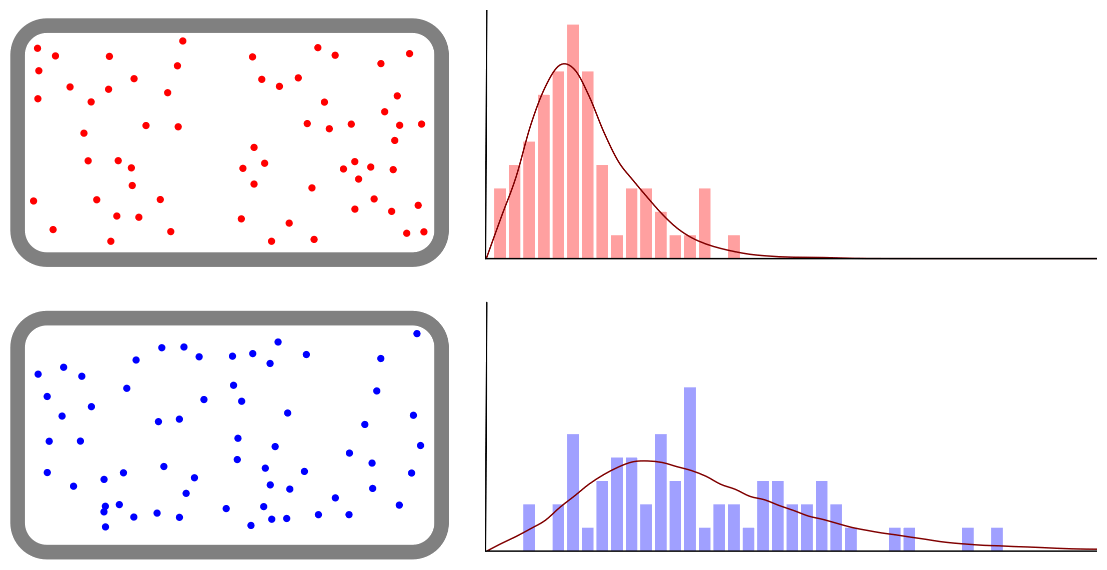
Diffusion of ink in cold and hot water
Particle movement according to temperature The matter around us is made up of tiny particles constantly moving. The particles move more actively as the temperature increases. Water particles move more actively in hot water than cold … more
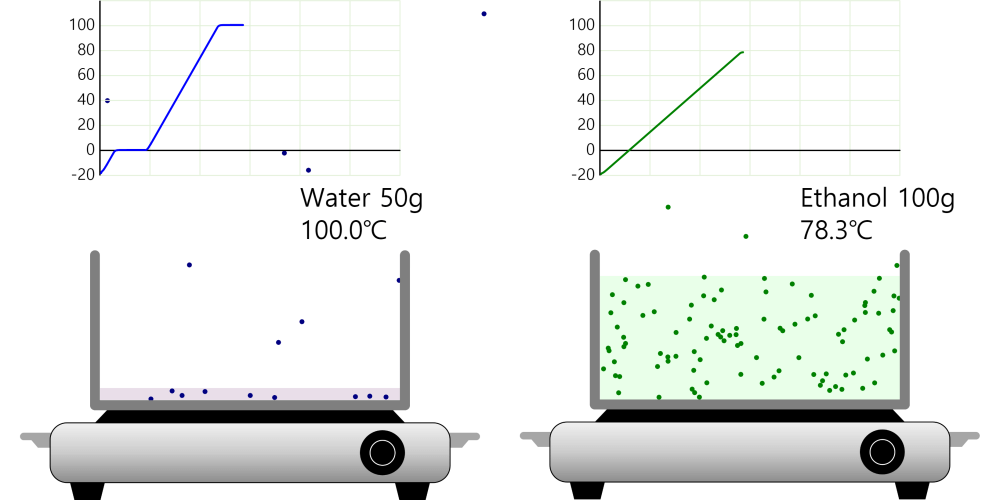
Boiling Point (Ice or Water, Ethanol)
Boiling point and properties of substances All water boils at 100°C when heated, even if it has a different mass. In this way, the same kind of substance has the same boiling point regardless of quantity. … more
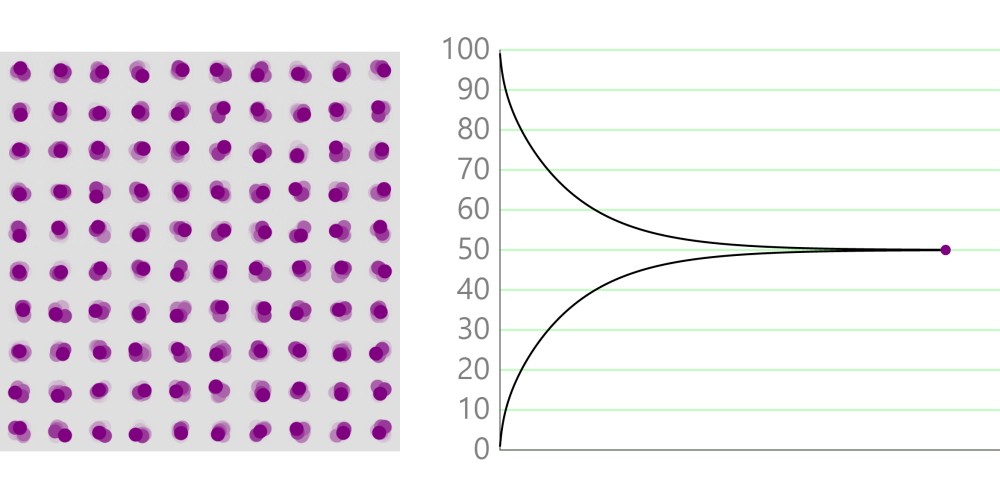
Particle Simulation of Thermal Conduction
About this simulation This simulation shows the particle’s temperature by its color.● Red means high temperature.● Blue means low temperature. We apply Hooke’s law between particles.(\(f=-kx\)) Thermal Conduction Conduction is a method of transfer of heat … more
Conduction
Conduction of Heat If a high-temperature object and a low-temperature object are attached to each other, the high-temperature object’s heat moves to the low heat by molecular motion. Examples of Conduction that can be seen around … more
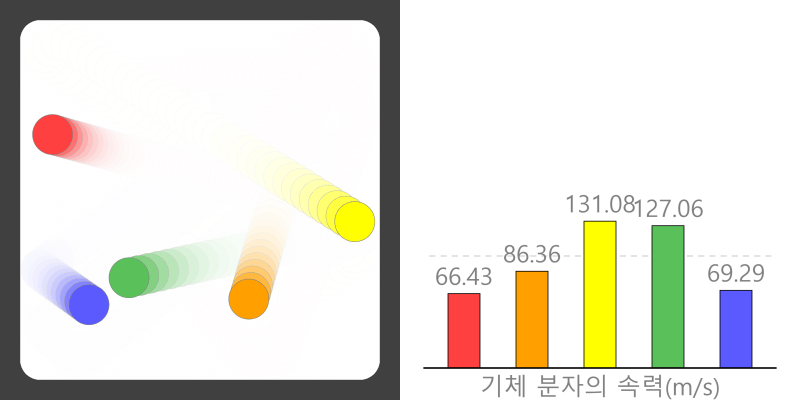
Root-Mean-Square Velocity of Gas Molecules (Vrms)
* The gas molecules in the above simulation are all of the same type, and the color of the molecules is for identification purposes only. Kinetic Molecular Theory of Gases ‘Kinetic Theory of Gases’ is a … more
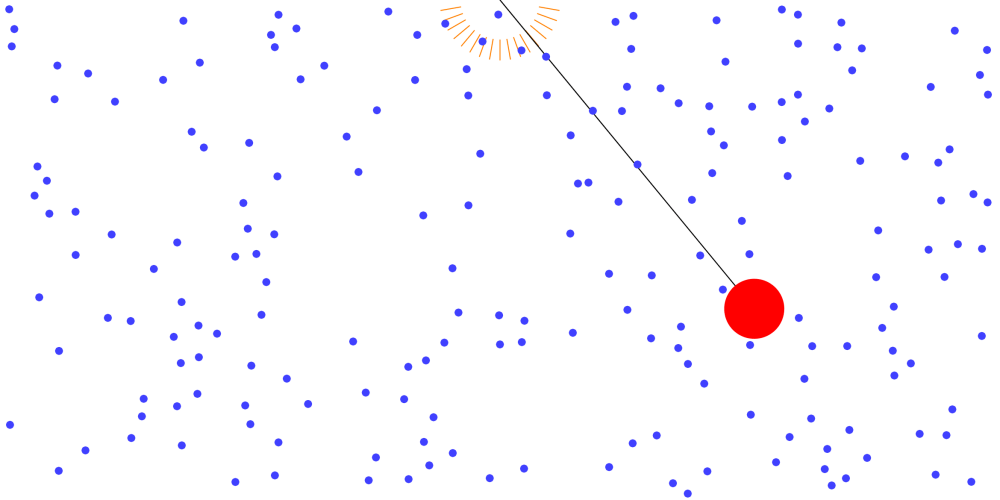
Where did the pendulum’s energy go? (The second law of thermodynamics and irreversible phenomena)
Where did the pendulum’s energy go? A pendulum that vibrates in the air continues to collide with air molecules in the process of vibrating. Air molecules get their energy from the pendulum. In this process, Air … more
The principle of air conditioner and refrigerator
The air conditioner The air conditioner is based on ‘evaporative heat'(a phenomenon that absorbs the surrounding heat when the liquid evaporates). The indoor unit suddenly expands the compressed refrigerant through a narrow hole. Then, the refrigerant … more
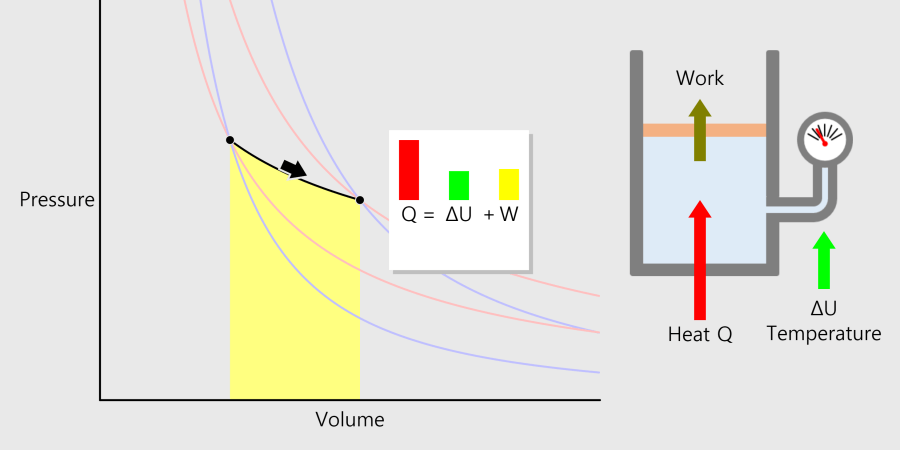
Pressure Volume Diagram
You can drag ‘start’ and ‘endpoint.’ Thermodynamics of Gas The gas changes its temperature while exchanging thermal energy with the outside and sometimes does work outside as it expands or contracts. The process of changing the … more
Temperature and Reaction Rate
Heat and molecular motion Every object is made up of atoms or molecules that are small particles that the eye can not see. These particles are constantly moved or oscillated themselves. This is called molecular motion. … more