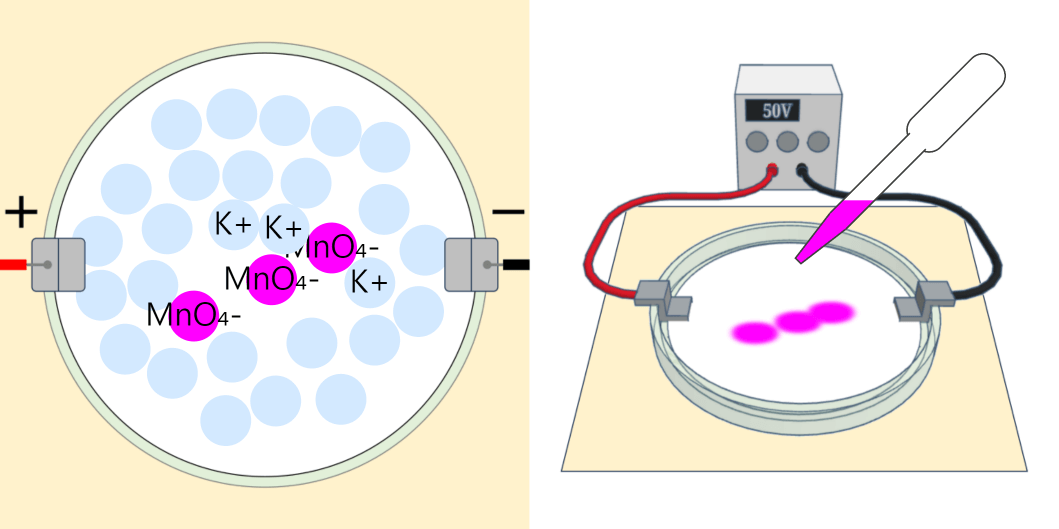
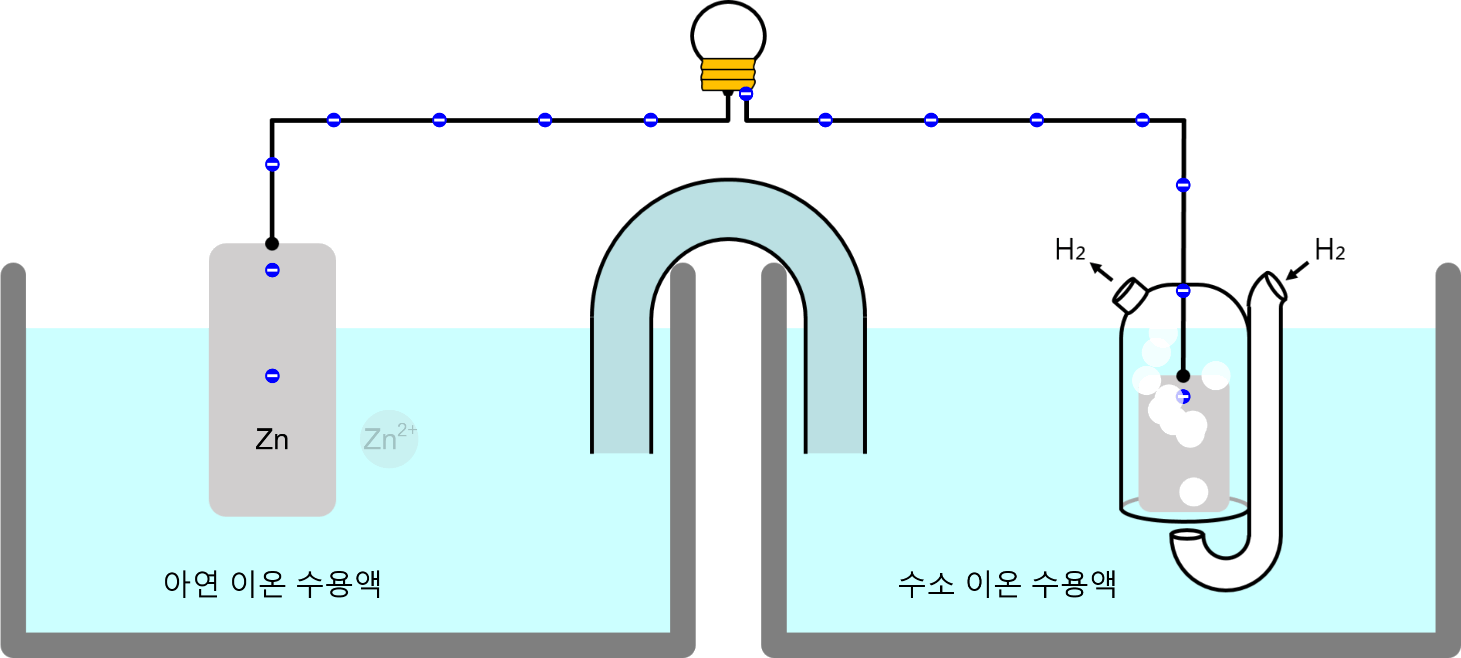
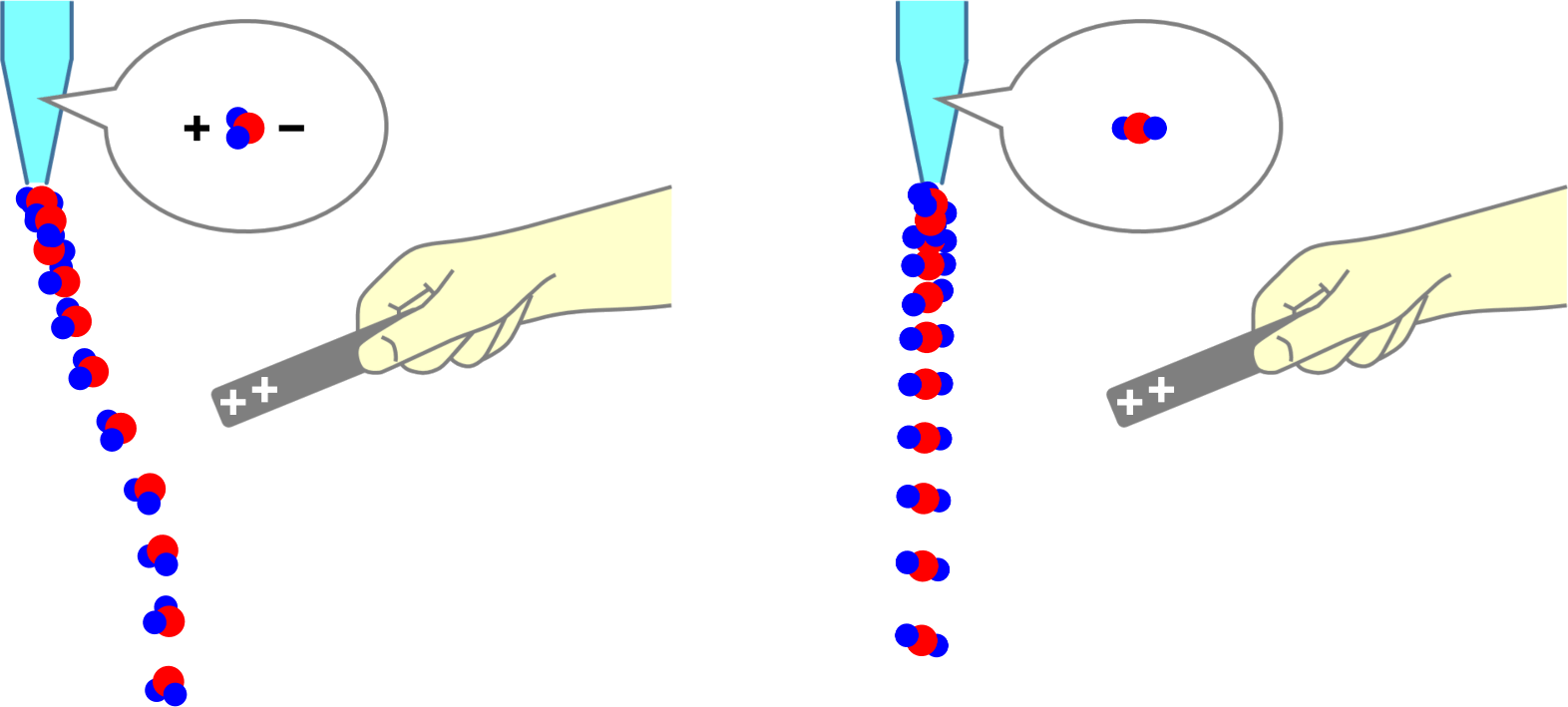
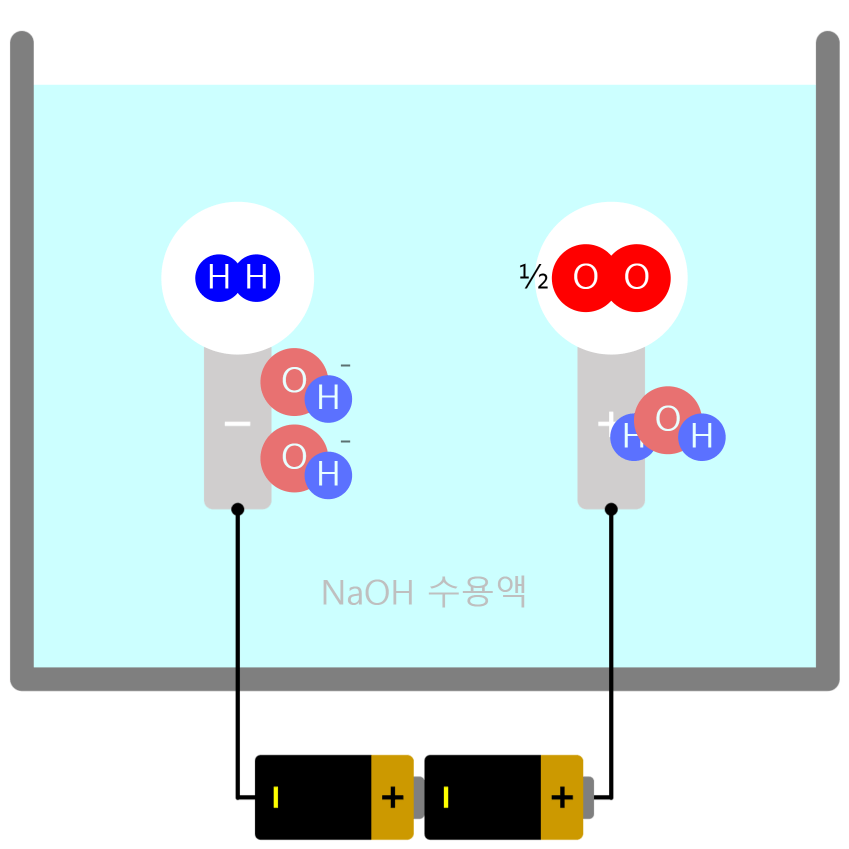
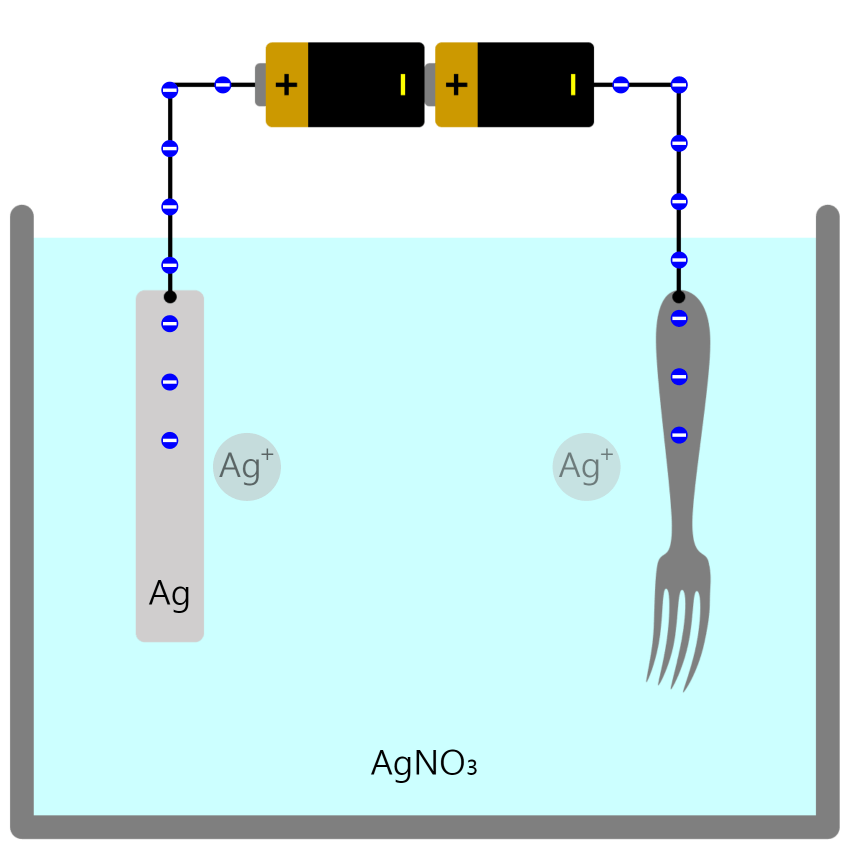
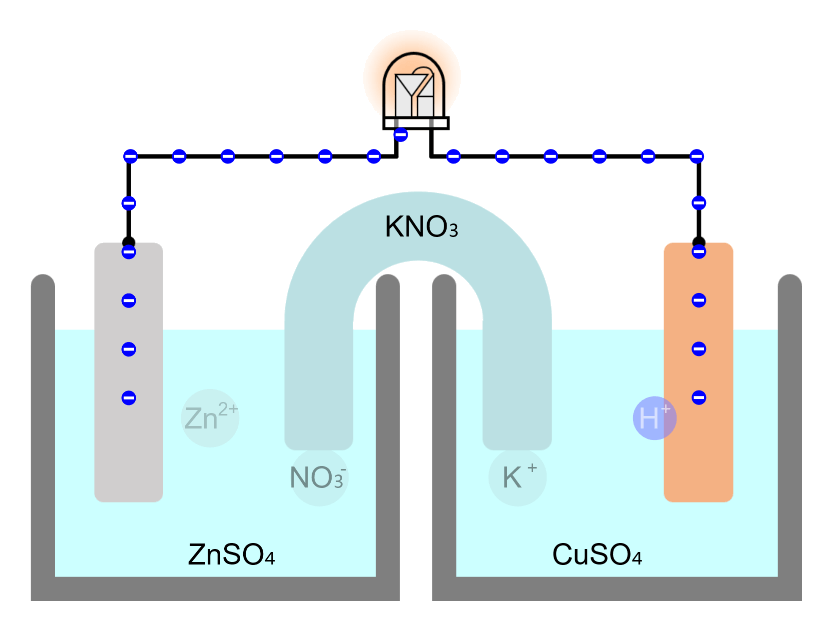
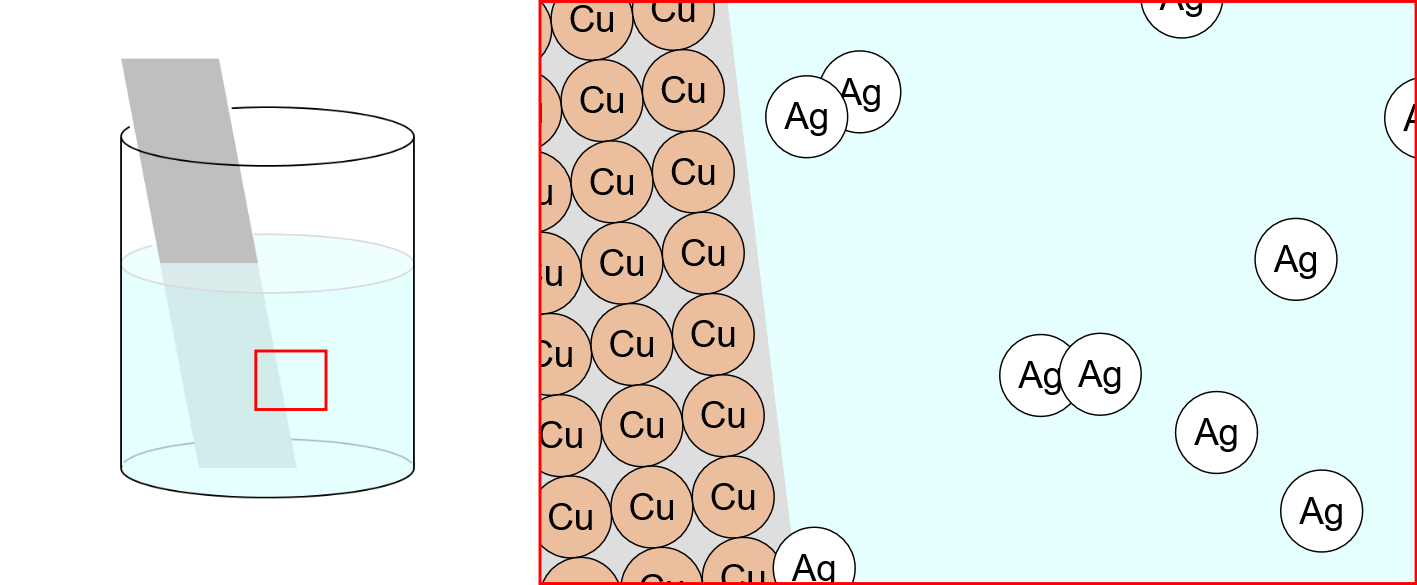
Movement of Ion particles
* You can move ion particles with the mouse. Movement of Ion particles Ions acquire electric charge by gaining or losing valence electrons, depending on the type of atom. Electric forces act between charged substances. A … more