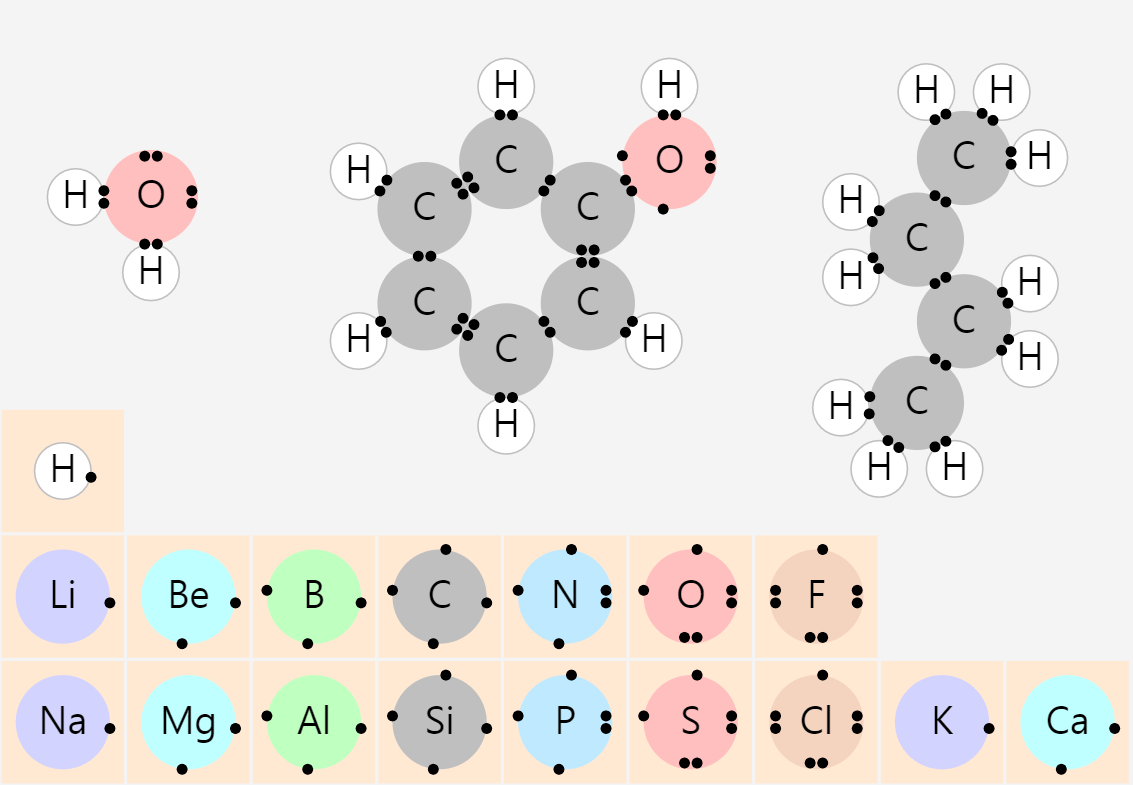
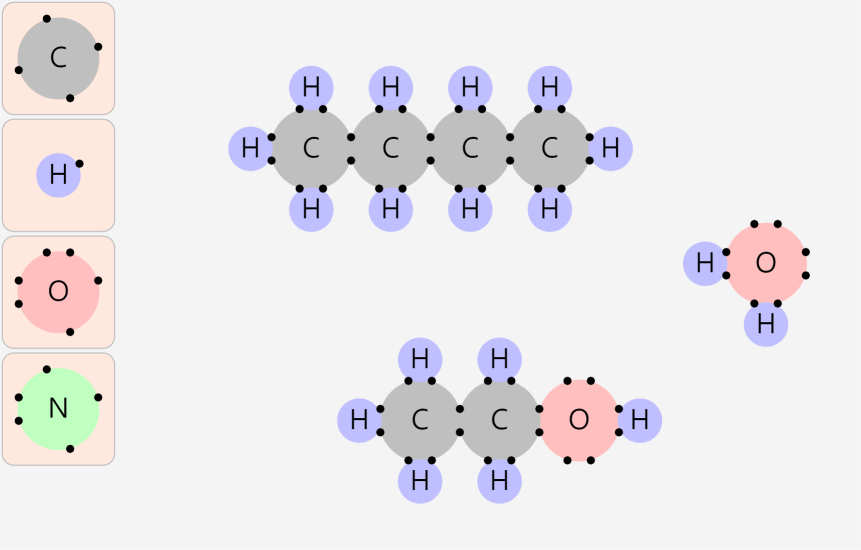
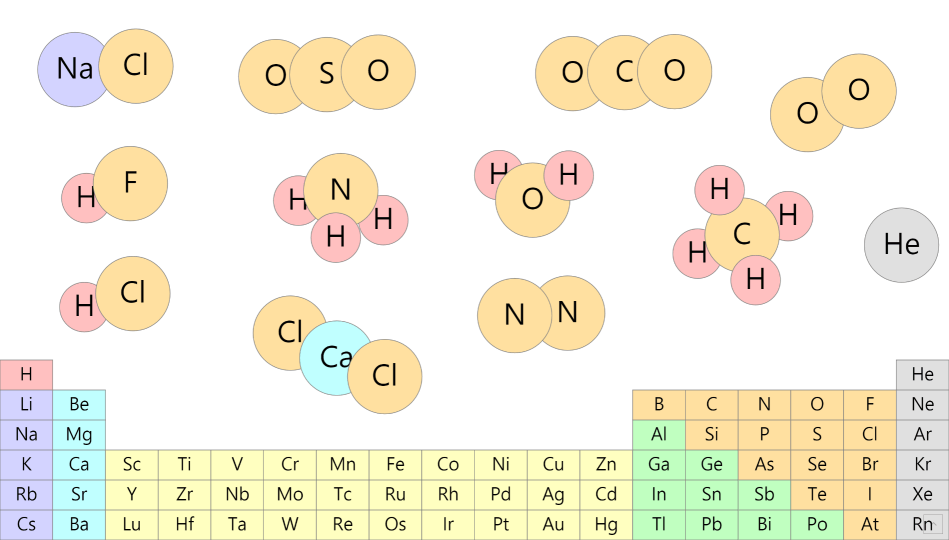
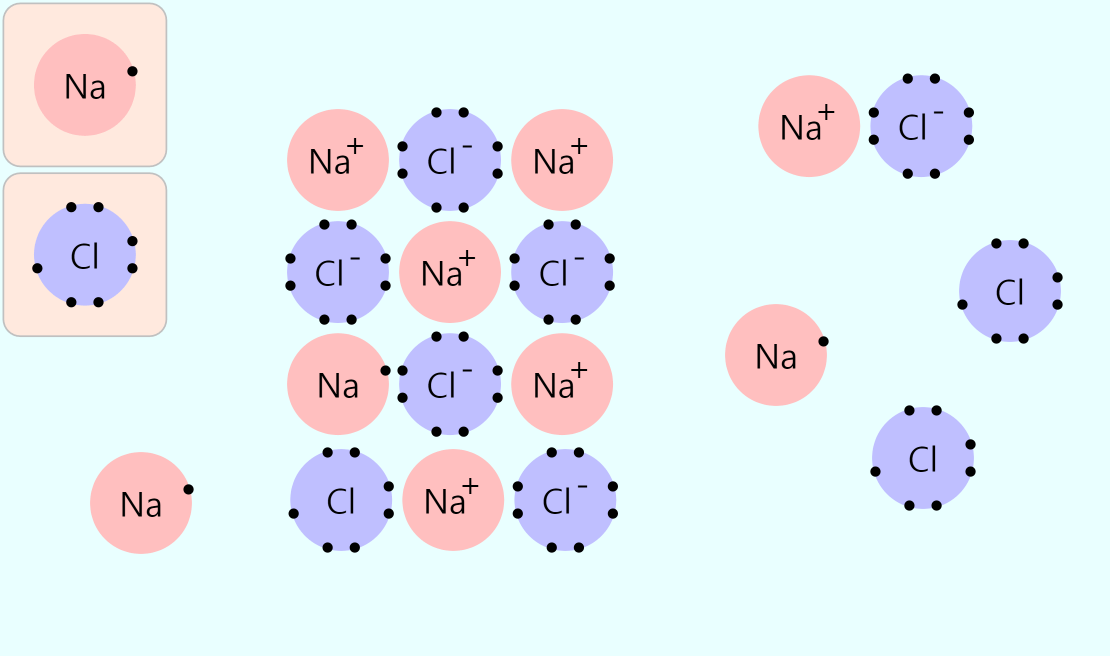
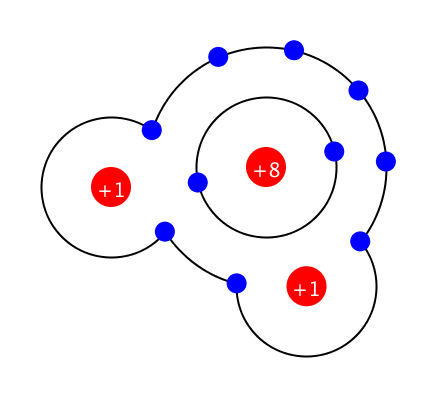
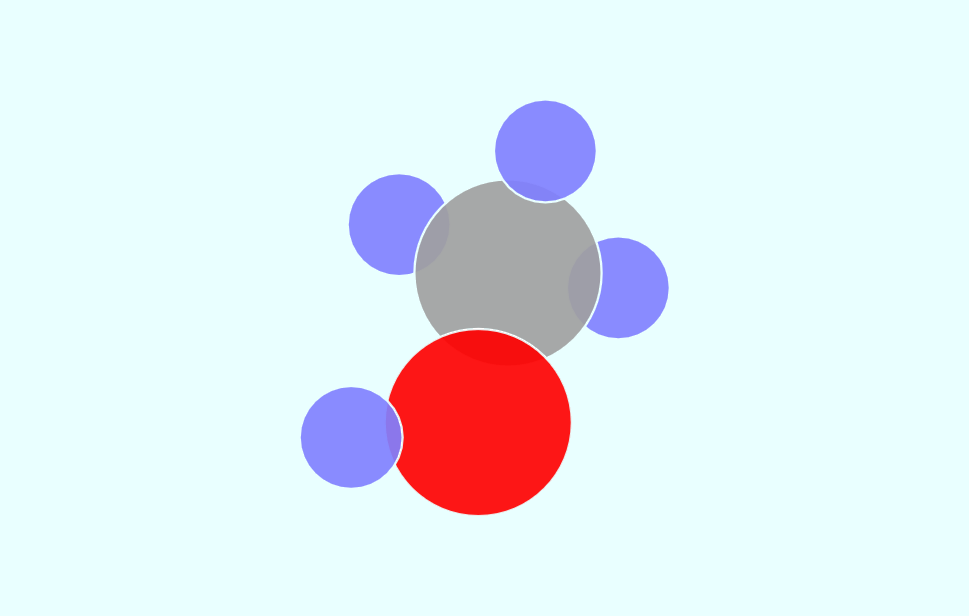
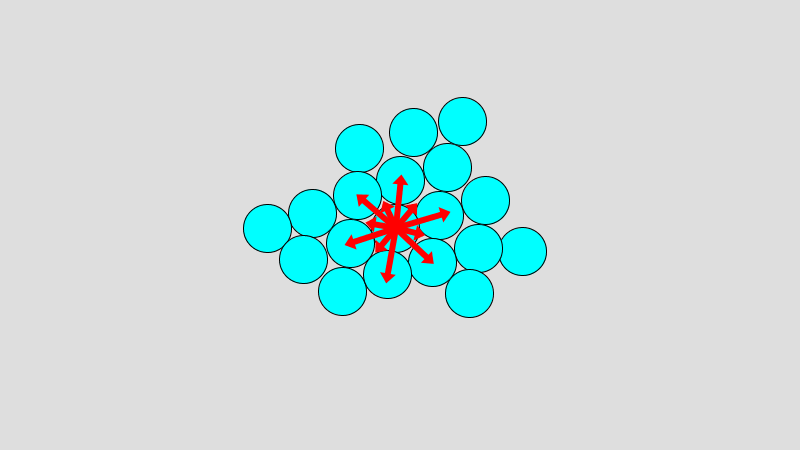
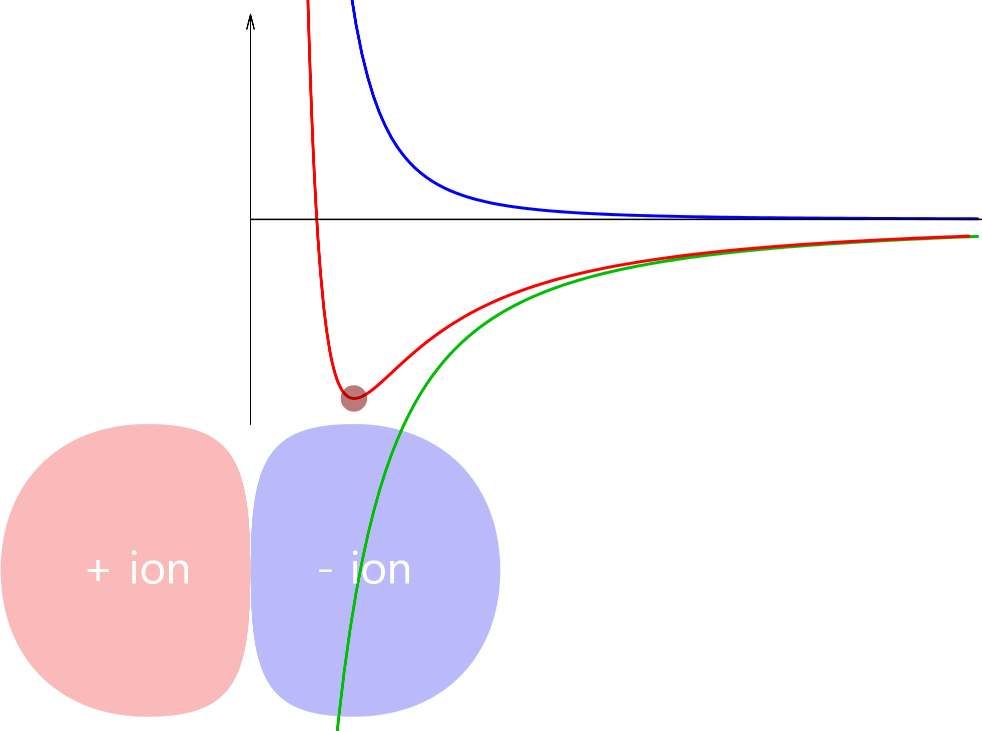
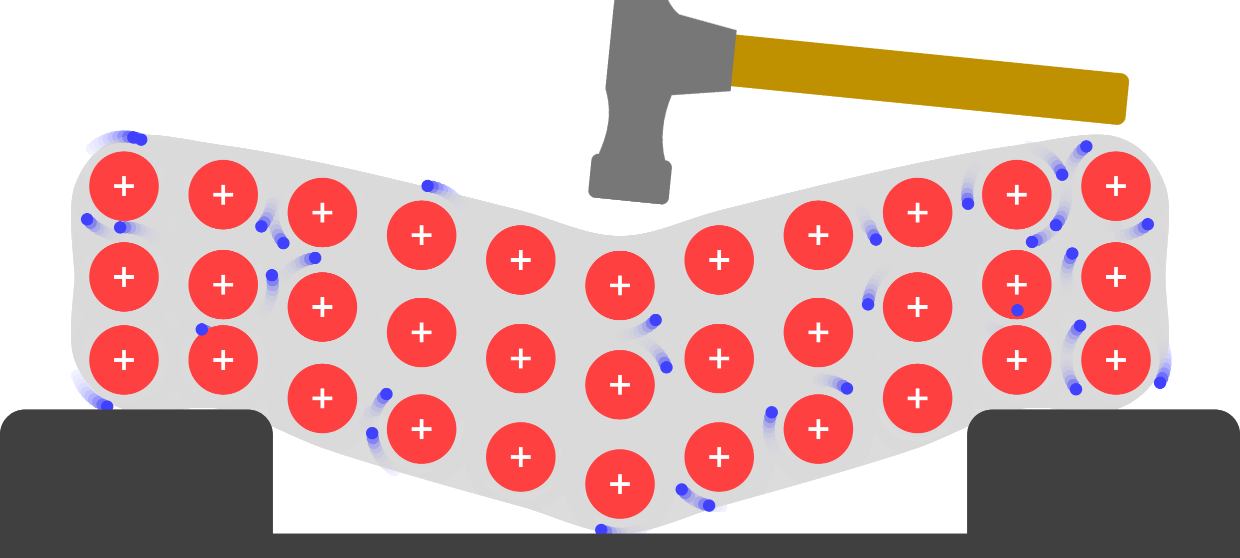
Simple Chemical Bonding Simulation
All atomic models showed only the electrons in the outermost shell. All chemical bonds are depicted as planar, but in reality, they can have three-dimensional structures. ‘In case of unstable, display in red’ may contain an … more